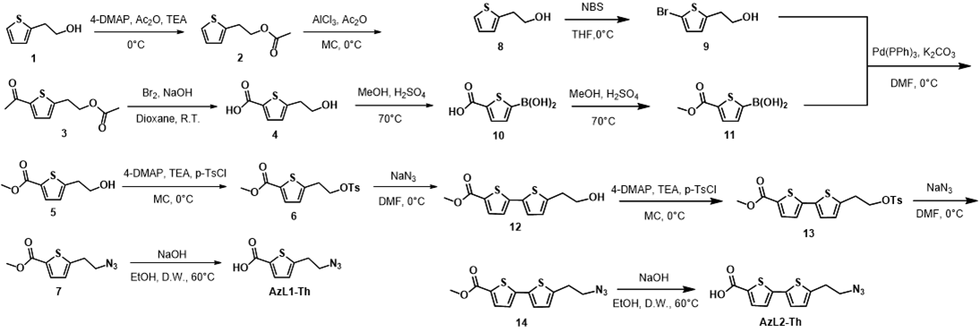
Synthesis of AzL1-Th / AzL2-Th ligand
Compound 7 (0.2g, 0.8mmol) / Compound 14 (0.3g, 1.2mmol) was added to a round-bottom flask, and the air was replaced with N2 by purging for 30 min. After dissolving compound 7 / compound 14 in ethanol, a solution of NaOH (0.1g, 3.2mmol / 0.4g, 10.6mmol) in 20ml/24ml water was added. The resulting mixture was heated to 60 °C and stirred overnight. After cooling to 26 ° C, the reaction was quenched with hydrochloric acid to a pH of 2. The reaction mixture was extracted with ethyl acetate and dried with magnesium sulfate. The crude product was purified via recrystallization from ethyl acetate and hexane. Thus, AzL1-Th / AzL2-Th was obtained (yield: 0.1 g, 77.3 % / 0.3 g, 82.8 %).
Figure. 1 Synthetic route of (a) AzL1-Th and (b) AzL2-Th
Figure. 2 Hole mobility of (a) AzL1-Th and (b) AzL2-Th. DFT modeling of (c) AzL1-Th, and (d) AzL2-Th. 1H NMR spectrum of synthesized (e) AzL1-Th and (f) AzL2-Th. 13C NMR spectrum of (g) AzL1-Th and (h) AzL2-Th.
Characterization
Hole mobility was recorded at a room temperature using a Keithley 4200 SCS (Tektronix, America).
Geometry optimization was performed using the DMol3 program in Materials Studio 4.4 package, which is a quantum mechanical code based on density functional theory (DFT).
Nuclear magnetic resonance spectra were measured using an JNM-ECZ500R (JEOL, JAPAN).